About us
A chemistry hub for your regulatory and non-regulatory compliant studies with radiolabelled substances and drug candidates
Novandi Chemistry are experts in de novo multi step custom synthesis of tritium, carbon-14 and stable labelled molecules.
Novandi Chemistry offers customers an overall responsibility from de novo radiosynthetic route development and synthesis of starting materials, to long-term storage, continuous quality control and third-party deliveries. Thus, Novandi Chemistry can guarantee high-quality compounds and simplify the logistics for studies with radiolabeled substances.
Starting materials and key intermediates are either manufactured in house or are provided by our customers. We welcome the opportunity to offer you a competitive quotation.

The Team
The staff at Novandi Chemistry has a strong portfolio of de novo radiosynthetic route development and successful fit for purpose label placement for ADME and imaging study success. In addition to radiochemistry, our combined expertise also includes synthetic chemistry, medicinal chemistry and radioligand development.

Transparency & Communication
We are always available for a TC to discuss your radiochemistry needs. Would you prefer to meet us and discuss your project on site? Then you are of course welcome to come by and pay us a visit. Don’t miss the opportunity to enjoy a cup of our famous java.
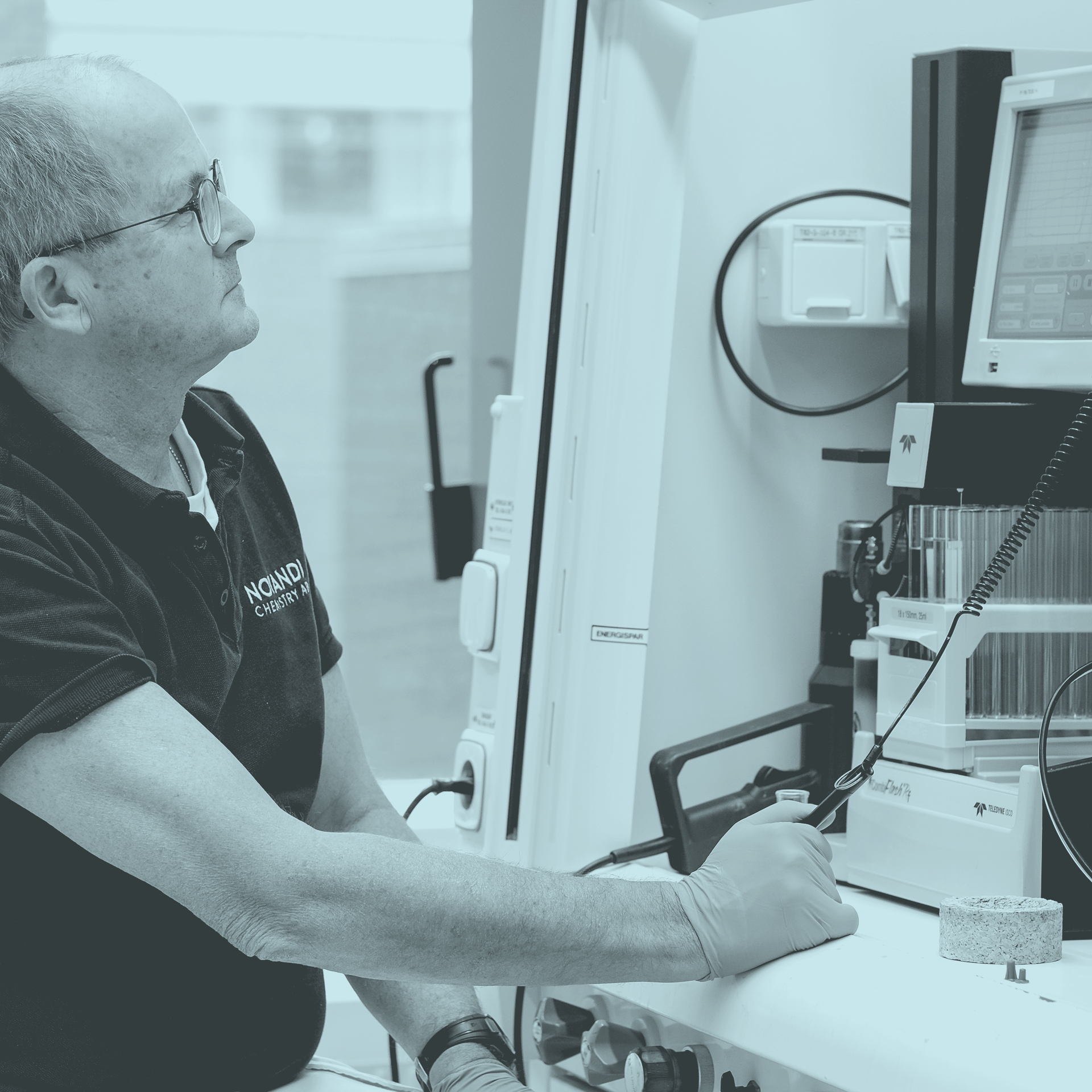
Instrumentation & Premesis
We operate a state of the art and fully equipped radiosynthetic and radioanalytical laboratory. With a chemical inventory comprising more than 15,000 chemical reagents and building blocks, we have a short starting distance for most synthesis assignments.

Quality Control
The quality of our products are guaranteed by analysis employing such methods as LC-MS, HPLC-Radiochemical flow, HPLC-UV, TLC-Radioscan, and NMR. Each radiolabeling and synthesis assignment is accompanied by a complete analysis report and a CoA.

Stability Study Service
Radiochemicals decompose over time and for all radiochemicals, the highest purity is obtained at the time of final purification. The shelf life depends on the speed of this process. To estimate the shelf life we measure the radiochemical purity of all new products at suitable intervals.

Storage Service
As part of our overall responsibility we can house your radiocompounds under adequate storage conditions. In combination with on demand third party shipping, your logistics around radiolabeled material is simplified.
Purification Service
During storage, decomposition may reduce the purity. Therefore it might be necessary to repurify a labeled compound to meet the quality required for your assay. By continuous quality control and purification prior dispatch, the highest possible quality of your substance is ensured.

Shipping & packaging
We regularly ship to North America, Europe and Asia. Most products are delivered in clear thick wall glass vials with conical bottoms for high recovery. Volatile products are shipped in prescored and flame sealed ampoules. Our products are accompanied by an AntiTipStandⒹ, which allows for safe and convenient dispensing and storage in a leak tight secondary container.

Certifications & licences
We are licensed and regularly inspected by the Swedish Radiation Safety Authority (SSM) that we fulfill all environmental and health regulations in accordance with the Swedish Radiation Protection Act.
We are also licensed by the Swedish Medical Products Agency to manufacture and sell controlled substances.